
Current Highlights:
Posted July, 2000 – Profiles of nitrous oxide (N2O) isotope fractions retrieved from balloon-borne observations
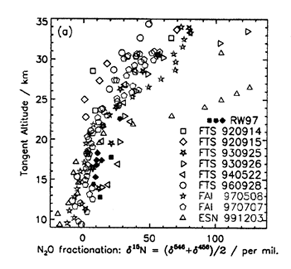
Nitrous oxide is released into the atmosphere from anaerobic processes in soils and oceans, and from industrial activities such as agricultural fertilization. The emitted N2O is eventually transported to the stratosphere where it is photolyzed and becomes the principal source of reactive nitrogen (NOx) in that region. The heavy isotopes of N2O are photolyzed slightly more slowly than the parent isotopomer (446), due to differences in their zero point energies, and this is predicted to cause the fraction of the heavy N2O to increase with altitude. Measurements of the N2O isotope fraction can reveal important insights into stratospheric transport and photochemistry. However, these measurements are difficult due to the low abundances of the heavier isotopes (< 1%).
An improved N2O linelist produced by Dr. R. Toth at JPL was used in conjunction with spectra taken from the JPL MkIV balloon (Dr. G. Toon and coworkers) to obtain accurate isotopic fractionations or the 456 and 546 isotopomers of N2O. The various isotopomers can be easily distinguished in the high resolution infrared spectra due to the red-shifting of transitions from the heavier isotopes. However, an accurate spectral deconvolution is only now possible with the aid of the new line list. Some of the various fractionation profiles are shown in the accompanying figure.
Posted July, 2000 - Low temperature kinetics of the chlorine monoxide (ClO) recombination reaction studied in laboratory
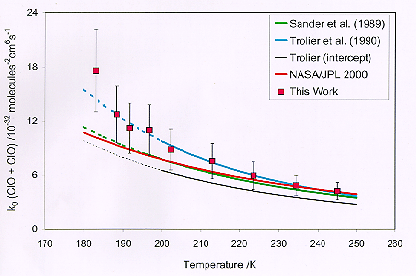
The large decreases in ozone concentrations in the Antarctic and Arctic stratosphere during the early spring have been linked to unusually high concentrations of the reactive chlorine oxide (ClO) species. Recombination of ClO (i.e. ClO+ClO+M®Cl2O2+M) has been recognized as a key reaction in the chlorine-catalyzed ozone destruction mechanism. The kinetics of the ClO self-reaction and the absorption cross sections of Cl2O2 have been studied using the technique of flash photolysis with UV absorption spectroscopy. Three independent monochromator / PMT monitoring channels were employed, two used to monitor the peak of the ClO (12,0) band at 275.2nm and the adjacent valley, and the third monitoring Cl2O2 absorption at shorter wavelengths. Concentrations of ClO could thus be determined via the differential absorption due to ClO permitting evaluation of the forward reaction rate. Experiments have been performed over the envelope of conditions relevant to the middle atmosphere. A plot of the third order reaction rate constant as a function of temperature is shown in the following figure. The study was conducted at JPL by Drs. S. P. Sander and W. Bloss. Prof. S. Nickolaisen of Cal State Los Angeles collaborated on the study.
Posted July, 2000 - Multi-platform campaign investigates ozone chemistry in the Arctic Stratosphere
The NASA-sponsored SAGE III Ozone Loss and Validation Experiment (SOLVE) has completed measurements of ozone chemistry occurring in the Polar Stratosphere during Winter and Spring 1999/2000. The researchers have observed this last winter to be one of the coldest on record and have documented ozone losses exceeding 60% over this time period. The new findings bolster the view that ozone depletion is sensitive to the spacial extent of temperatures below the formation threshold for polar stratospheric clouds as well as the abundance of industrially produced chlorine and bromine compounds.
The large U.S. sponsored effort involved four flights of a high altitude balloon maintained by JPL and numerous flights of the ER-2 and DC-8 NASA aircraft. Integrated onto the three platforms were dozens of instruments, from JPL and many other institutes, that measured the abundance of ozone and other atmospheric gases and particles. JPL researchers provided the mission with the following instruments: ALIAS/Dr. C. Webster, ALIAS II/Dr. D. Scott, JLH/Dr. R. Herman, Ozone/Dr. J. Margitan, MkIV/Dr. G. Toon, SLS/Dr. R. Stachnik, MTP/Dr. M. J. Mahoney as well as modeling support /Dr. R. Salawitch.
Posted July, 2000 – Airborne study blazes new trails into the effects of aviation and rocket exhaust in the atmosphere
The Atmospheric Chemistry of Combustion Emissions Near the Tropopause (ACCENT) mission is a multi-agency sponsored effort to evaluate the roles of aircraft and rocket exhaust in perturbing ozone chemistry and modifying aerosols and clouds. The mission was based at Ellington Field in Houston, Texas and air sampling flights were conducted in April, 1999 and again in September, 1999. The science payload for ACCENT consisted of 25 instruments that were capable of measuring a wide variety of gases and particles. The instruments were mounted on a NASA WB-57 aircraft (see figure below). During the ACCENT deployments, the WB-57 aircraft successfully characterized background aerosols on transit flights between Houston and San Jose, Costa Rica, intercepted plume wakes of Atlas IIAS, Delta II, and Athena II launches from the Cape Canaveral and Vandenberg facilities, and sampled aged aircraft plumes over the Dallas, Texas area. Preliminary results from the mission reveal a large diversity in the chemical composition of aerosols in the upper troposphere and lower stratosphere, large local ozone loss in the wakes of rockets, and readily identifiable perturbations in upper tropospheric concentrations of nitrogen oxides due to aircraft traffic. Detailed analysis of the ACCENT data is ongoing. JPL researchers served the mission in a number of capacities. Dr. R. Friedl was the co-mission scientist. Drs. R. Herman and M. J. Mahoney were the principal investigators on the water vapor and temperature profiler instruments, respectively.

NASA WB-57 in flight. Picture courtesy of NASA Johnson Space Flight Center
[Back to Top]
[Back to the
Atmospheric Chemistry Homepage]
Author: Randall R. Friedl
Page Design: Aaron B. Milam